Influence of Biofilms in Wound Healing
Medline has collaborated with Professor Steven Percival to address the issue of biofilms and their role in wound healing. With more than 25 years of experience, Professor Percival is an expert on biofilms, infection prevention, microbiology and medical devices. He has worked in academia and at start-ups, global multinationals, the NHS and the CDC.
Biofilms are everywhere. They are composed of microorganisms that are attached to a surface and become encased with polysaccharides and proteins. They can be found on surfaces in the kitchen, on the surface of ponds, in showerheads, on your teeth and even in chronic wounds. In healthcare, biofilms are thought to be responsible for increased infection in wounds, and they delay wound healing.1 Scientific knowledge, research and understanding of biofilms and their role in health and disease have increased significantly over the last few years.
What is a biofilm?
A biofilm is defined as a community of microorganisms that are attached to a surface, or each other, and encased within a matrix of sticky polysaccharides, proteins and extracellular DNA called extracellular polymeric substance (EPS).² Biofilms can be located on virtually any inert or living surface where they represent an entity that is difficult to eradicate. This is because a biofilm is recalcitrant to both antimicrobial agents and the host’s immune system.³ Consequently, biofilm eradication represents an important research area in chronic diseases and infections.
How does a biofilm form?
Biofilm formation is a very complex multi-step process, where microbial growth changes from the planktonic (free-floating) state to the sessile (attached) state.
The first step in the formation of a biofilm is microbial attachment (adhesion) to a surface. Once attached, the microorganisms increase in number and colonisation begins. Further microbial growth occurs, different microbes are recruited into the biofilm and the biofilm grows even bigger, generating more extracellular material and eventually forming a 3D structure. A mature biofilm generally contains 10 per cent microbial cells and 90 per cent extracellular material.
Throughout the biofilm formation process, microbes are continually shed and then colonise other ‘virgin’ surfaces, akin to metastasis in a benign tumour. As with any living thing, reproduction is essential to the lifecycle, and the biofilm is no exception. Dispersal enables biofilms to spread and colonise other sites within the human body and within the environment.²
Where can biofilms form?
Biofilms can be found on living surfaces, non-living surfaces and in liquids. They are present in nature within rivers and streams but can also be found in man-made environments, like hotel and hospital water systems, waste water systems and domestic showers. Often, their formation is aided by the presence of an aqueous solution. Consequently, they are frequently found floating as microbial aggregates within liquids.
Biofilms are found in our bodies—in our oral cavity, on our skin and in our digestive tracts. They generally have a beneficial commensal relationship with the host and help protect the host from infection and disease. However, they can also take on a detrimental role, leading to conditions such as gum disease and tooth decay.
Interestingly, biofilms also play a significant role in mining and metal ore extraction. They have also been shown to help clean up contaminated groundwater and soils. (4)
Role in Wound Healing
Biofilms can develop in both acute and chronic wounds, as they are moist and highly nutritious environments. Numerous studies on humans and animals have shown that biofilms impede wound healing.5 Biofilms should be taken seriously by healthcare professionals, as they can increase infection risks and also lead to delays in wound healing.
Biofilm formation in a wound
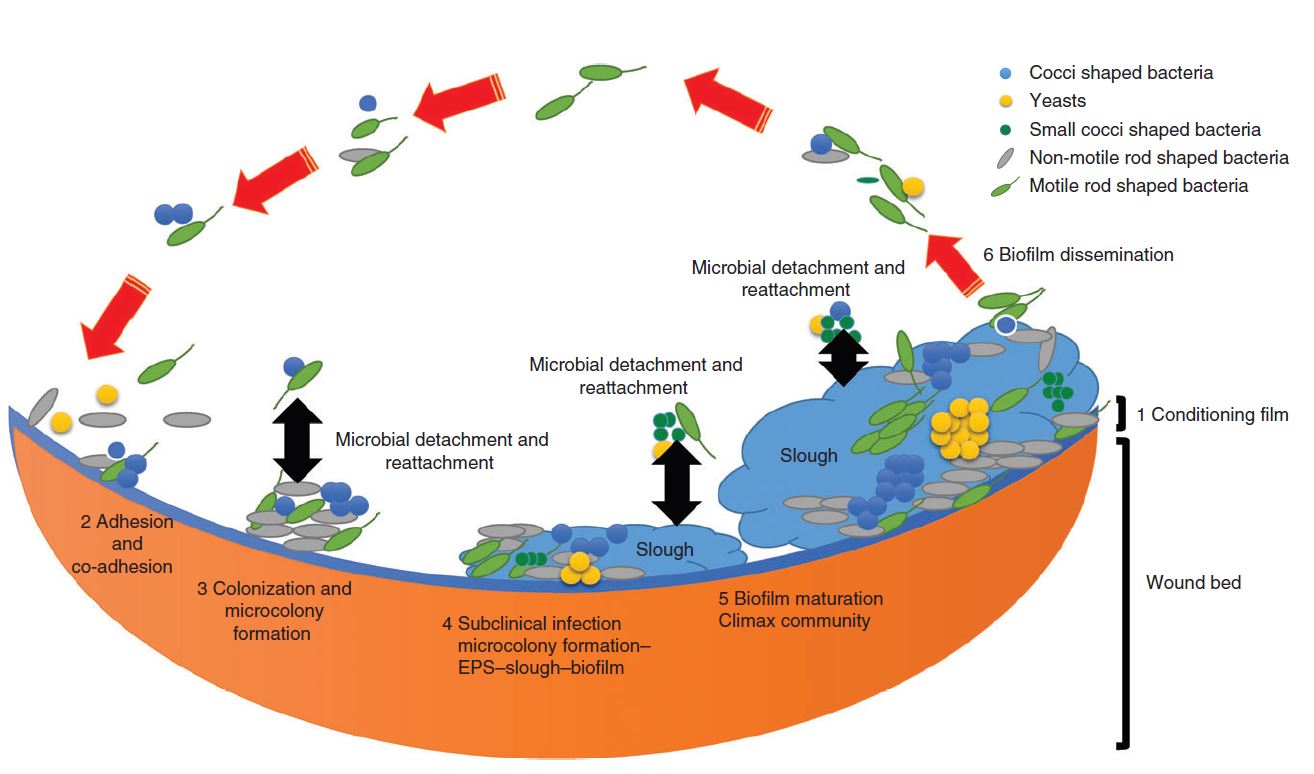
Figure 1 illustrates the six stages of biofilm formation in a wound.
- Stage 1: A conditioning film (protein) forms within milliseconds and is present on any virgin surface.
- Stage 2: The conditioning film plays a key role in biofilm formation, as microbes actually attach first to this film and not the surface itself.
- Stage 3: Attachment can be reversible or irreversible. If irreversibility is achieved, then the microbes can start to multiply and form micro-colonies.
- Stage 4: This stage is the first instance in which biofilms can be microscopically identified. As the biofilm matures, more EPS is produced, which strengthens its securement to the wound surface.
- Stage 5: The microbiology of the biofilm begins to stabilise, resulting in microbial homeostasis.
- Stage 6: Microbial detachment and reattachment then occur, which can be serious in terms of infection control and cross-contamination.1
Figure 1: Stages in the development of a mature biofilm in the wound bed1
Delay in wound healing
Acute wounds taking more than six weeks to heal are classified as chronic wounds. Examples of chronic wounds include surgical wounds and various types of ulcers, all of which are very susceptible to infection. A conceptual study in 20046 suggested that biofilms were the underlying cause of prolonged infection and non-healing of most chronic wounds. This conjecture was confirmed by a different study in 2008,7 which reported that 60 per cent of chronic wounds sampled had a biofilm present.1 These biofilms are responsible for stimulating and maintaining wound inflammation, increasing infection and delaying wound closure. Furthermore, additional research has indicated that dry wounds are also at risk for developing biofilms, and wound dressings can even encourage the growth of biofilms.8 Table 1 shows the wound-microbiology-biofilm continuum and describes the stages of infection and delays in wound healing.
| Stage | Microbiological state | Clinical indicators |
| 1 | Contamination – microorganisms are present but are transient. | No obvious clinical indicators. No damage to tissue. |
| 2 | Adhesion and colonization – multiplication of microbes. Biofilm to planktonic ratio in favour of planktonic phenotypic state. | No obvious clinical indicators, signs or symptoms. No damage to tissue. |
| 3 | Subclinical or biofilm infected – often referred to as critically colonized. This stage involves multiplication of microorganisms. Biofilm to planktonic ratio in favour of biofilm phenotypic state. | Wound healing delayed. No obvious clinical indicators and signs of infection or symptoms. ‘Quiet’ inflammation. |
| 4 | Local infection – microbial growth, multiplication and invasion into host tissue causing a host immune response. | Clinical indicators and signs of infection, such as inflammation, redness, swelling, warmth, pain, cellulitis, increased exudate. Damage to tissue. |
| 5 | General infection/sepsis. | Fever, increased heart rate, increased breathing, confusion. |
Table 1: The wound-microbiology-biofilm continuum.1
Impact on the hospital and patient
Complex wounds represent a significant economic burden on healthcare systems, and delays to the healing of these wounds can make this burden exponentially worse for care facilities and patients alike.9
Addressing and Preventing Biofilms
Preventing biofilms and appropriately treating them if they occur can help eliminate or reduce additional monitoring, treatment, costs and recovery complications associated with delayed or stagnated wound healing.
Detection
|
Criteria used by Clinicians to determine if a wound is infected: |
Criteria suggesting that biofilms may be responsible for surgical-site infections¹: |
|
Pain |
Evidence of pathogenic micro-organisms |
|
Abscess |
Direct examination of tissue that demonstrates evidence of aggregated (microcolonies) microbes in an extracellular polymeric substance matrix |
|
Cellulitis |
Confirmation of infection supported by clinical markers |
|
Raised body temperature |
Recalcitrance to antibiotic and antimicrobial treatment despite evidence of susceptibility of the isolated microbes in the planktonic state |
|
Necrotic tissue |
Culture-negative samples irrespective of confirmation of microbes by culture-independent protocols |
|
Slough |
Ineffective host clearance and evidence of inflammatory cells |
|
Putrid smell |
Recurring state of chronic inflammation |
|
Friable tissue that bleeds |
Recurring local infection |
|
Yellow discharge and slime |
Infection returning after an antimicrobial intervention has been stopped |
|
Excessive inflammation |
|
All of these features can be easily seen or measured. But with biofilms, which aren’t visible to the naked eye, identification becomes much more complex. Biopsies are vital in diagnosing biofilms, but this unfortunately is not always possible. In these cases, healthcare professionals have to rely on a variety of clinical, biological and therapeutic indicators. These include:
- Inflammation (similar to that of normal wound infection)
- Recalcitrance to the present standard protocols of care
- Ineffective antimicrobial performance
- Recurring infections
Prevention and best practices
A patient’s immune system, if immunocompromised, can rarely fight off a biofilm on its own. But when trying to do so, it overproduces polymorphonucleocytes and white blood cells, which results in chronic inflammation and delayed wound healing. Studies have shown that many presently available antimicrobials and wound dressings do not effectively manage or eradicate biofilms effectively. This has resulted in a growing focus on this wound care topic and the development of innovative antibiofilm agents such as tetrasodium EDTA.
To minimise susceptibility to biofilms and reduce wound infection risks, the following strategies are recommended:
- Minimise the risk of cross-contamination (especially for open wounds)
- Use antimicrobial prophylaxis (especially if the area contains opportunistic pathogens)
- Employ appropriate aseptic techniques during wound management
- Prepare the skin properly before a surgery
- Choose dressings for acute wounds carefully, making sure that they can:
- Absorb exudate
- Reduce pain and discomfort
- Allow for visual inspection of the wound
- Protect and maintain newly formed tissue
- Support a moist wound environment
- Immobilise microbes and prevent them from entering the wound bed.1
Clearly, the purpose of a wound dressing is to aid in timely wound healing and closure. Table 2 shows the traditional expectations of a wound dressing and also how these expectations have been updated to accommodate the prevention and control of biofilms.10
| Pre-biofilm concept criteria | Post-biofilm concept criteria |
| Facilitation of debridement (when necessary) | Extracellular polymeric substance breakdown (antibiofilm) |
| Maintenance of thermal insulation | Microbial sequestration and immobilisation within the wound dressing |
| Reducing scar formation | Antimicrobial |
| Removal of excess wound exudate | De-sloughing/cleaning |
| Does not shed fibres and is non-toxic | Absorption of excessive exudate |
| Non-adherent, comfortable with intimate contact | Matrix metalloproteinase modulation |
| Manage wound bioburden | Moist environment known to promote healing |
| Enhancement of cell proliferation |
Table 2: Criteria for an effective wound dressing
Risk factors
One of the main risk factors for biofilm development is wound healing by secondary intention. Naturally, the longer a wound is open, the higher the risk of microbial contamination. These types of wounds require careful monitoring. Another risk factor is the wound dressing itself, as it can be a breeding ground for microbes. To combat this risk, frequent dressing changes may be necessary.1
Plurogel: Concentrated Surfactant Technology
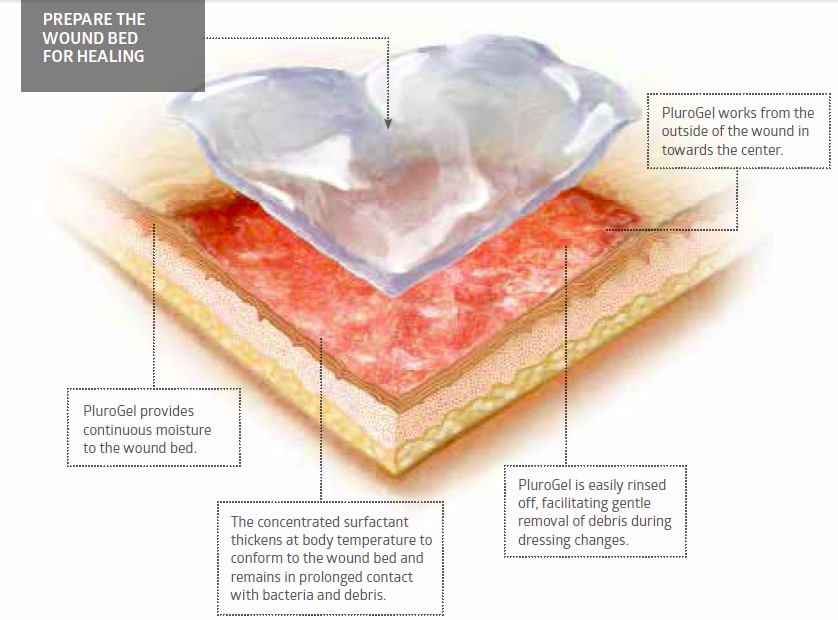
PluroGel is a unique burn and wound dressing. It is a stable, viscose gel comprised of a concentrated surfactant. PluroGel provides an optimally moist wound healing environment and facilitates the separation of loose, non-viable tissue, maintaining a clean wound bed.
By design, PluroGel is non-toxic, biocompatible and 100 per cent water-soluble. Its unique inverse thermal dynamic properties allow it to thicken in a warm wound bed and conform to the wound. This ensures the surfactant formulation stays in constant contact with the necrotic tissue and debris in the wound bed. PluroGel manages the microenvironment of the wound by solubilising debris and creating a rinsing effect on a molecular level. This disrupts mature biofilm and is a preventative measure against the formation of new biofilm.
Click here to learn more about PluroGel burn and wound dressing or here to learn more about PluroGel with PSSD.
Technology
PluroGel is made of a non-ionic surfactant known as a poloxamer. Surfactant monomers contain a hydrophilic head and a hydrophobic tail. Under a process called micellization, a large number of surfactant molecules organise and orient themselves into a group, and then form a micelle. In this flexible, spherical micelle structure, the surfactant molecules align to bear a hydrophilic surface and a hydrophobic centre.
Image of a micelle
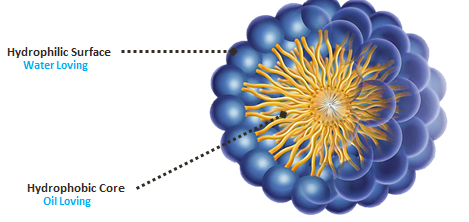
By binding with the water in the emulsion, the hydrophilic surface facilitates the micelle’s movement in the wound environment, creating a constant cleansing effect on a molecular level. The hydrophobic core is attracted to oil-based debris and, being a flexible space, this core is able to expand to trap oil-based debris, solubilising it over time. The micelle structure changes over time too, collapsing and expanding constantly to trap the wound debris and create a rinsing action on a microscopic level.
Research
Evidence that surfactants are effective against biofilm activity is growing. Topical antimicrobials, such as surfactants, have been recommended to prevent or delay attachment of planktonic microbes and eradicate any disrupted or dispersed biofilm.
In a recent study ‘Efficacy of a surfactant-based wound dressing on biofilm control’ published in the Wound Repair Regeneration journal, the efficacy of PluroGel and PluroGel PSSD against biofilms was tested. Results showed complete death of microorganisms within a biofilm using the antimicrobial surfactant-based wound dressing. Interestingly, the non-antimicrobial surfactant-based dressing could disrupt existing biofilms by causing biofilm detachment.10
Related Products
References
1 Percival S. L., Importance of biofilm formation in surgical infection. Published online in Wiley Online Library, 2017.
2 www.rroij.com. Accessed 2 March 2018.
3 http://bacteriality.com/2008/05/biofilm/. Accessed 2 March 2018.
4 http://waterandhealth.org/safe-drinking-water/drinking-water/biofilms-good-bad-2/. Accessed 2 March 2018.
5 Percival S. L., McCarty, S. M., & Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Advances in Wound Care, 373–381, 2015.
6 Percival S. L., Bowler PG. Biofilms and their potential role in wound healing. Wounds 2004; 16: 234–240.
7 James GA, Swogger E,Wolcott R, Secor P, Sestrich J, Costerton JW et al. Biofilms in chronic wounds. Wound Repair Regen 2008; 16: 37–44.
8 Percival S. L., Restoring balance: biofilms and wound dressings. Journal of Wound Care, Vol. 27, No. 2, February 2018.
9 Evelyn Walter, Karin Schalle, Alexandra Lazic-Peric, Marco Voit, Sophie-Christin Hausberger, IPF Institute for Pharmaeconomic Research, Vienna, Austria. Cost-Effectiveness of PluroGel® - a new Micelle-matrix-based dressing with 1% silver sulphadiazine – in the management of non-healing wounds.
10 Percival S. L., Mayer, D. M., & Salisbury, A.-M. P. (2017). Efficacy of a surfactant-based wound dressing on biofilm. Wound Repair Regeneration, 1-7.
Plurogel is a medical device of class IIa non-sterile, Plurogel PSSD is a medical device of class III non-sterile, intended to be used by healthcare professionals. Before use, consult instructions and precautions on the corresponding labelling.![]() 0373 Istituto Superiore di Sanita
0373 Istituto Superiore di Sanita![]() STUDIO AMBIENTE S.r.l ., Via Monte Baldo, 4 37062 Dossobuono di Villafranca di Verona (VR), Italia
STUDIO AMBIENTE S.r.l ., Via Monte Baldo, 4 37062 Dossobuono di Villafranca di Verona (VR), Italia