MDR Preparedness
Medline is working hard to prepare for the EU’s Medical Device Regulation (MDR). And our goal is to have all MDR certificates in place for all of our existing products by 2023, one year ahead of schedule.
What is the MDR?
The MDR is legislation that replaces the EU’s current Medical Device Directive (MDD). The MDR will apply to all medical device manufacturers who have goods for sale on the European market. It came into force on 25 May 2017. We are now in the transition period where both legal frameworks can be applied. However, as of 27 May 2024, only devices with an MDR certificate may be sold.
What are the differences between the MDD and MDR?
The MDR builds upon the MDD and adds new requirements for medical goods for sale on the European market. Its overarching goal is to increase the safety and efficiency of the EU medical device market. Compared to the MDD, the MDR:
- Increases clinical investigation requirements and manages risk to ensure patient safety
- Reinforces surveillance and management of the entire MD life cycle
- Improves transparency and traceability
- Reduces ambiguity with clear classifications and definitions.
The MDR also presents more stringent requirements for the designation of notified bodies, with increased control and monitoring by the national competent authorities and the European Commission.
What does the MDR mean for Medline and the medical device industry?
The MDR will affect all medical devices sold in the EU, whether they are manufactured in the EU or imported from outside the EU. That means, all devices will need to be recertified under the MDR with new CE certificates obtained by 27 May 2024. Medline is well on its way to be ready for the MDR.
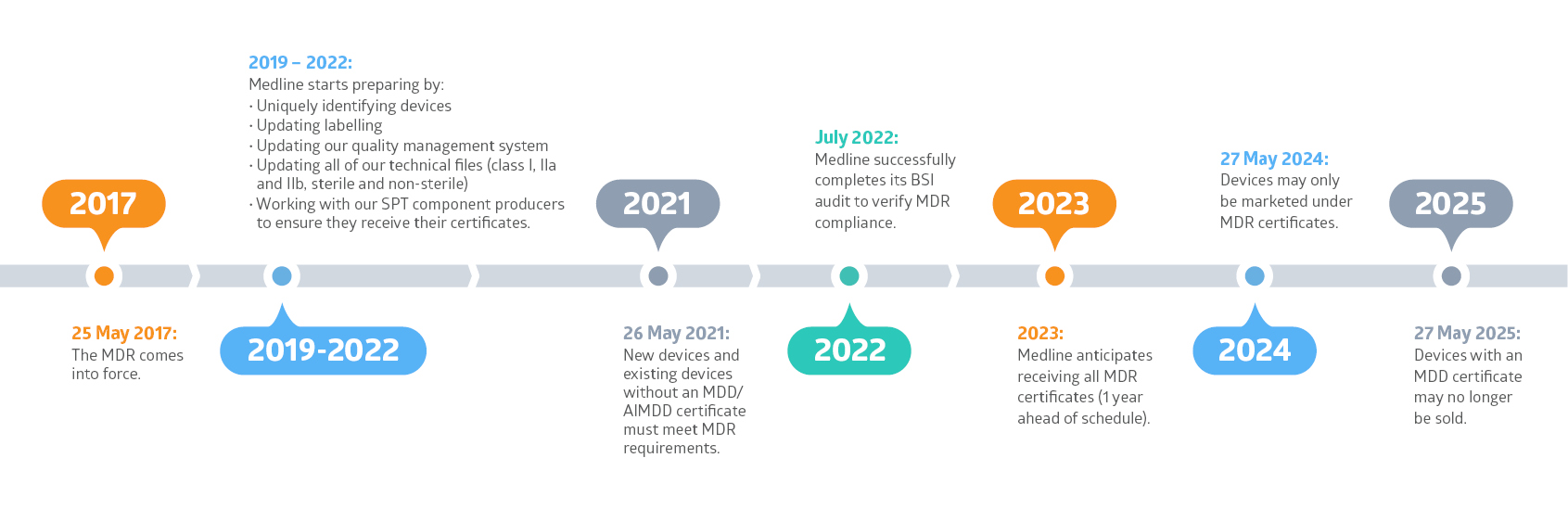

Medline Continuing to Prepare for the MDR Thanks to Successful BSI Audit ( Published 27.7.2022)
Preparing for the New EU Medical Devices Regulation (MDR) (Published 17.12.2019)
